Non-Disclosure/Confidentiality Agreement Services
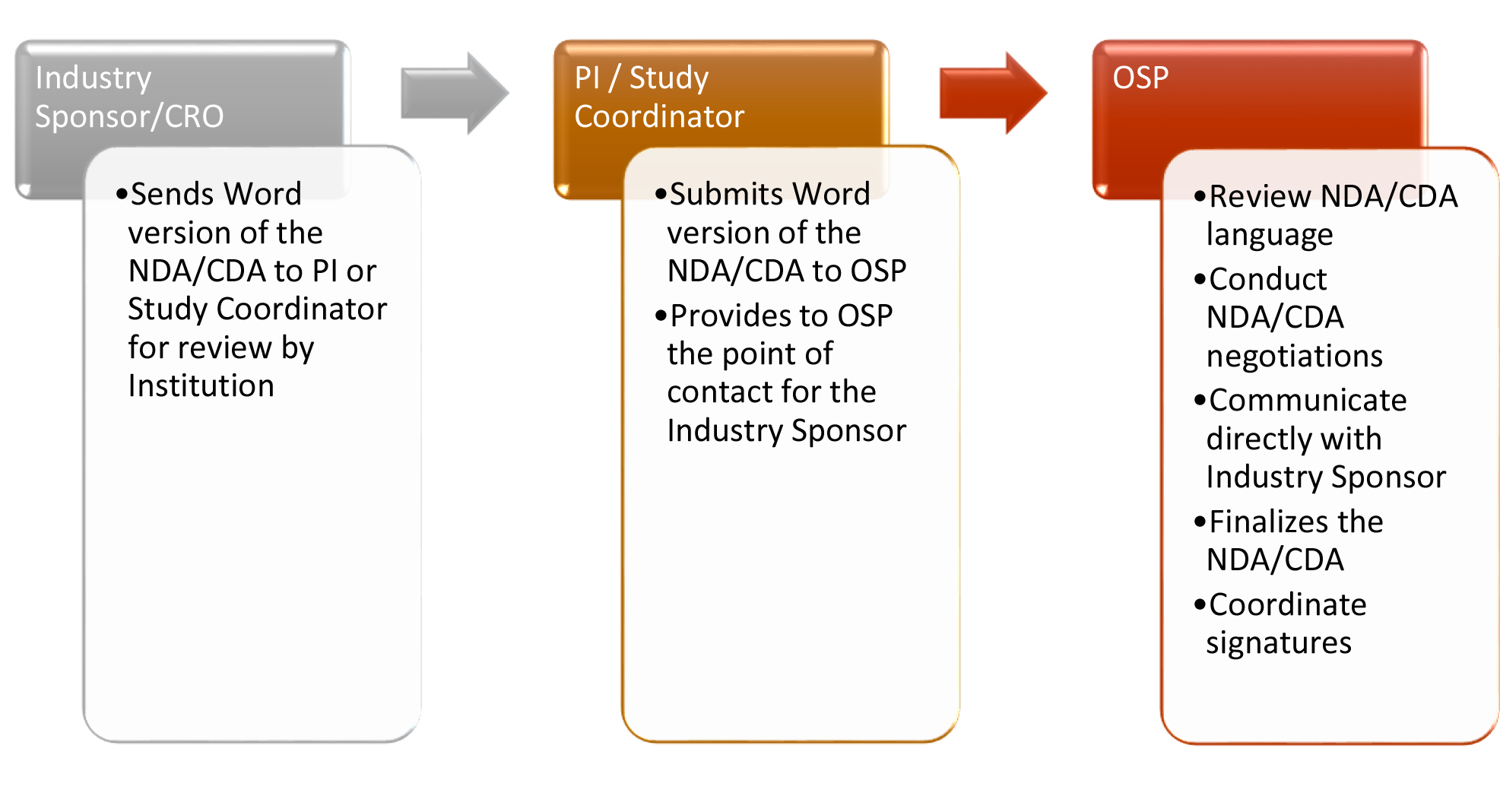
Non-Disclosure/Confidentiality Agreements (NDA/CDA) are legal documents that ensure confidentiality of proprietary information that an industry sponsor reveals to the principal investigator (PI). A signed, study-specific NDA/CDA is generally required before a sponsor will provide its proprietary information (e.g., a study protocol) to a PI.
The terms and conditions of a NDA/CDA will be negotiated in accordance with TTUHSC El Paso policies. The Office of Sponsored Programs (OSP) will negotiate with the Sponsor or Contract Research Organization (CRO) until the agreement is acceptable to both parties. Establishing the terms of the CDA is critically important since these terms most likely will define the provisions of the study-specific industry clinical trial agreement.
OSP Point of Contact:
Deborah Martinez
915-215-4405
deborah.c.martinez@ttuhsc.edu
NDA/CDA Workflow Process